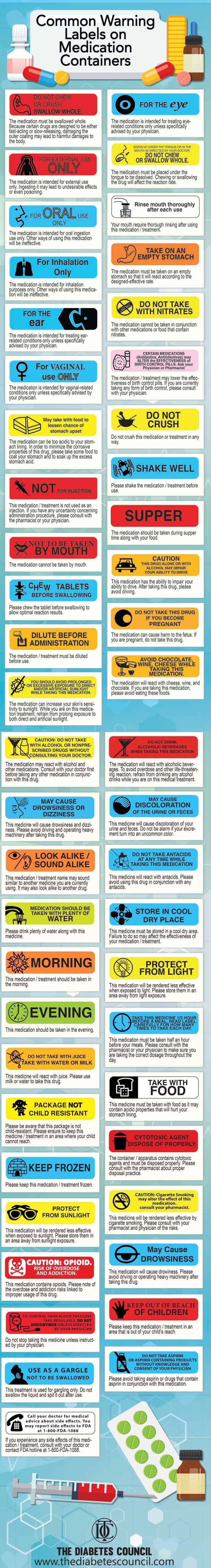
39 cautionary and advisory labels for dispensed medicines
Therapeutic Goods Regulations 1990 - Legislation Feb 12, 2019 · Required Advisory Statements for Medicine Labels means the advisory statements specified by the Minister by legislative instrument under subsection 3(5A) of the Act. sample includes part of a sample. serious, in relation to a form of a disease, condition, ailment or defect, means a form of the disease, condition, ailment or defect that is: The Drugs and Cosmetics Rules, 1945 - Indian Kanoon Apr 07, 2016 · An application for an import licence shall be made to the licensing authority in Form 8 for drugs excluding those specified in Schedule X, and in Form 8A for drugs specified in Schedule X, either by the manufacturer himself having a valid wholesale licence for sale or distribution of drugs under these rules, or by the manufacturer’s agent in India either having a valid licence under the ...
British National Formulary - Wikipedia The British National Formulary (BNF) is a United Kingdom (UK) pharmaceutical reference book that contains a wide spectrum of information and advice on prescribing and pharmacology, along with specific facts and details about many medicines available on the UK National Health Service (NHS).

Cautionary and advisory labels for dispensed medicines
Poisons Standard June 2021 - Legislation May 26, 2021 · Human medicines required to be labelled with a sedation warning. List of human medicines required to be labelled with a warning regarding their sedation potential. Appendix L. Requirements for dispensing labels for medicines. Requirements applying to labels attached to medicines at the time of dispensing. Appendix M Cautionary and advisory labels | About | BNF | NICE List of the 30 cautionary, warning and advisory labels applied to the medications used in the BNF, as found in appendix 3 of the printed edition. Poisons Standard June 2022 - Legislation May 31, 2022 · Human medicines required to be labelled with a sedation warning. List of human medicines required to be labelled with a warning regarding their sedation potential. Appendix L. Requirements for dispensing labels for medicines. Requirements applying to labels attached to medicines at the time of dispensing. Appendix M
Cautionary and advisory labels for dispensed medicines. Community Pharmacy - SlideShare Aug 09, 2016 · 4. Alternative medicines- In some countries, pharmacists supply traditional medicines and dispense homoeopathic prescriptions Checking symptoms of minor aliments- pharmacist can supply a non-prescription medicine, with advice to consult a medical practitioner if the symptoms persist for more than a few days. Alternatively, the pharmacist may ... Poisons Standard June 2022 - Legislation May 31, 2022 · Human medicines required to be labelled with a sedation warning. List of human medicines required to be labelled with a warning regarding their sedation potential. Appendix L. Requirements for dispensing labels for medicines. Requirements applying to labels attached to medicines at the time of dispensing. Appendix M Cautionary and advisory labels | About | BNF | NICE List of the 30 cautionary, warning and advisory labels applied to the medications used in the BNF, as found in appendix 3 of the printed edition. Poisons Standard June 2021 - Legislation May 26, 2021 · Human medicines required to be labelled with a sedation warning. List of human medicines required to be labelled with a warning regarding their sedation potential. Appendix L. Requirements for dispensing labels for medicines. Requirements applying to labels attached to medicines at the time of dispensing. Appendix M


















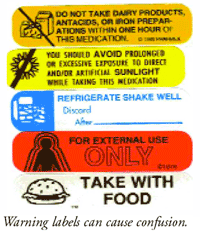
![PDF] Improving prescription drug warnings to promote patient ...](https://d3i71xaburhd42.cloudfront.net/e087ba4ebaa8ed043bf22bd2ab63292d6a05b9d4/2-Figure1-1.png)





Post a Comment for "39 cautionary and advisory labels for dispensed medicines"