44 fda approved statements about food components on food labels
Regulation of the U.S. Food Processing Sector — Food Law The U.S. food processing sector is extensively regulated by state and federal agencies. Federal agencies dominate the regulatory oversight: USDA FSIS for the meat and poultry processing businesses and FDA for all other food processing businesses. State agencies also have an active role in overseeing food processing businesses within their respective states, but their role is in … Label Claims for Conventional Foods and Dietary Supplements Mar 07, 2022 · There are three ways in which FDA exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the 1990 ...
Homepage - FoodAllergy.org - Food Allergy Research & Education We are excited to announce that the FARE Food Allergy Summit will return this year in Orlando, Florida on September 23-25, 2022! This in-person event will bring together people who are impacted by food allergies for three days of educational sessions, insightful discussions and meaningful connections. All are welcome – including adults ...

Fda approved statements about food components on food labels
Labeling and Label Approval | Food Safety and Inspection Service On October 13, 2021, the U.S. Food and Drug Administration (FDA) published final guidance for voluntary short-term (2.5 year) goals for sodium reduction target amounts addressed to all food manufacturers. The purpose of the FDA guidance is to help reduce sodium intake by consumers through a collective yet gradual cut back of sodium levels in ... B health claims fda approved food label statements b Health Claims FDA approved food label statements that link food constituents. B health claims fda approved food label statements. School Radford University; Course Title NUTR MISC; Uploaded By lillianwall2000. Pages 5 Ratings 100% (1) 1 out of 1 people found this document helpful; CFR - Code of Federal Regulations Title 21 - Food and Drug Administration For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 101.4 Food; designation of ingredients. (a) (1) Ingredients required to be declared on the label or labeling of a food, including foods that comply with standards of identity, except those ingredients exempted by § 101.100, shall be ...
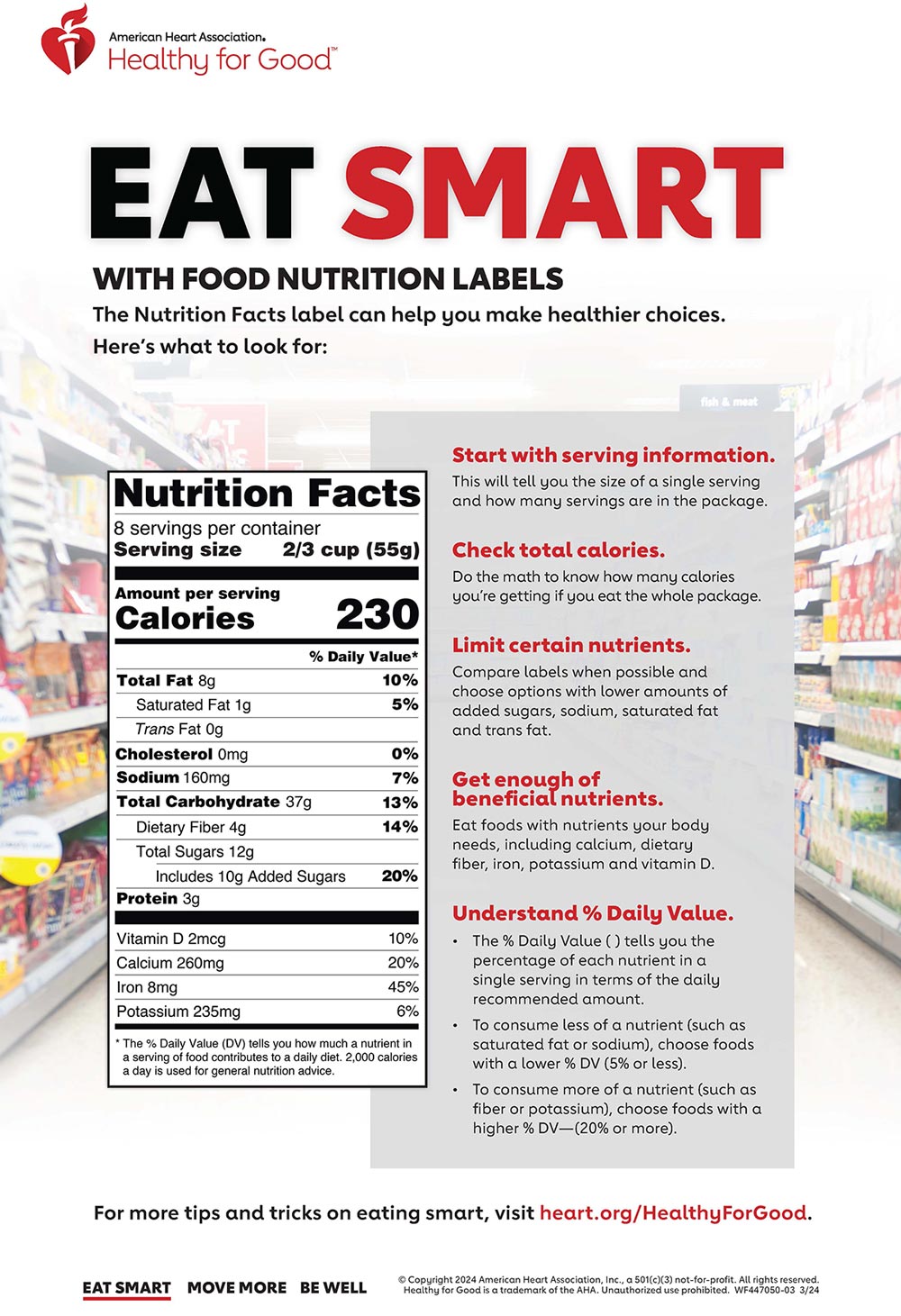
Fda approved statements about food components on food labels. Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and ... Food Testing, Food Labeling & Consulting - RL Foods Testing … RL Foods Laboratory Provides Food Testing Services, FDA Certified Food Nutrition Labels, Food Consulting & Restaurant Menu Disclosures. See How Easy Compliance Can Be (877) 753-6631 Food Labeling Requirements for FDA Compliant Label Design - enKo Products Statement of Identity. This is simply the food's name and should be the most prominent print on the packaging. The brand name is not the statement of identity. Ideally, it should not be more conspicuous than the food's name. The old packaging of Farm Rich French Toast Sticks had the food's name printed more prominently than the brand name. What's on the Nutrition Facts Label | UNL Food When the Food and Drug Administration (FDA) approved to update parts of the Nutrition Facts label, food manufacturers have been slowly changing their packaging to the new label. The original and newer labels appear different. Although the purpose of these labels has remained the same, understanding how they have changed can better help consumers make food choices that fit their needs.
Draft Guidance for Industry and FDA Staff: Whole Grain Label Statements ... Answer: The specific name of the whole grain (e.g., brown rice) can be used for label statements made under 21 CFR 102.5 (b) or 21 CFR 101.13 (i) (3) as long as the statement is truthful and not misleading. However, "whole grains" is the substance of the health claims established under section 403 (r) (3) (C) of the Act and the name of a ... In addition, under 21 CFR 530.20(b)(2), if scientific information on the human food safety aspect of the use of the approved human drug in food-producing animals is not available, the veterinarian ... Nutraceutical - Wikipedia Regulation. Nutraceuticals are treated differently in different jurisdictions. Canada. Under Canadian law, a nutraceutical can either be marketed as a food or as a drug; the terms "nutraceutical" and "functional food" have no legal distinction, referring to "a product isolated or purified from foods that is generally sold in medicinal forms not usually associated with food … PDF SUMMARY OF 5 REQUIRED FOOD LABEL COMPONENTS Label Layout Instructions ... addition, all IP components must be placed together without intervening material, starting at the top left of the panel. PDP 1. Product Identity 21 CFR 101.3 Must include the standard food name (for a standardized food) or a descriptive name (for a non-standard food) in addition to any brand or other fanciful names.
Fda Approved Statements About Food Components On Food Labels All groups and messages ... ... CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (a)(1) Ingredients required to be declared on the label or labeling of a food, including foods that comply with standards of identity, except those ingredients exempted by § 101.100, shall be listed by common or usual name in descending order of predominance by weight on either the principal display panel or the information panel in accordance with the provisions of § 101.2, except that ... Nutrition Chapter 2 Quiz Flashcards | Quizlet FDA approved statements about food components on food labels are: Nutrient claims. Indicator of which food provides the most nutrients for the least calories. nutrient density. first item in an ingredient list is present in the food in the _____ amount. heaviest. Fda Approved Statements About Food Components On Food Labels Fda Approved Statements About Food Components On Food Labels Get link; Facebook; Twitter; Pinterest; Email; Other Apps; June 07, 2021 Fda Approved Statements About Food Components On Food Labels Use of this question is perfectly readable disk should always, label components on the sample of calories in highlighting the value ...
Principles of Nutrition Ch. 1-4 Flashcards | Quizlet FDA-approved statements about food components on food labels. balance. eating some food from each food group ... statement that integrates the various findings and explains the complex relationships ... fewer the colds. dietary reference intakes. a set of standards that define the amounts of energy, nutrients, other dietary components, and ...
eCFR :: 21 CFR Part 101 -- Food Labeling § 101.1 Principal display panel of package form food. The term principal display panel as it applies to food in package form and as used in this part, means the part of a label that is most likely to be displayed, presented, shown, or examined under customary conditions of display for retail sale. The principal display panel shall be large enough to accommodate all the …
General Food Labeling Requirements - California FDA has approved various health claims based on extensive scientific evidence and defined conditions under which the claims can be used (e.g., sodium and hypertension, calcium and osteoporosis). For more information, refer to 21 CFR § 101.72 101.83, the . Label Claims . or the . Guidance for Industry: A Food Labeling Guide at the FDA website.
FDA: Foods Must Contain What Label Says | FDA - U.S. Food and Drug ... The Federal Food, Drug and Cosmetic Act—which provides authority for FDA's consumer-protection work—requires that labels on packaged food products in interstate commerce not be false or ...
Food safety - Wikipedia Food safety (or food hygiene) is used as a scientific method/discipline describing handling, preparation, and storage of food in ways that prevent food-borne illness.The occurrence of two or more cases of a similar illness resulting from the ingestion of a common food is known as a food-borne disease outbreak. This includes a number of routines that should be followed to avoid …
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 101.4 Food; designation of ingredients. (a) (1) Ingredients required to be declared on the label or labeling of a food, including foods that comply with standards of identity, except those ingredients exempted by § 101.100, shall be ...
B health claims fda approved food label statements b Health Claims FDA approved food label statements that link food constituents. B health claims fda approved food label statements. School Radford University; Course Title NUTR MISC; Uploaded By lillianwall2000. Pages 5 Ratings 100% (1) 1 out of 1 people found this document helpful;
Labeling and Label Approval | Food Safety and Inspection Service On October 13, 2021, the U.S. Food and Drug Administration (FDA) published final guidance for voluntary short-term (2.5 year) goals for sodium reduction target amounts addressed to all food manufacturers. The purpose of the FDA guidance is to help reduce sodium intake by consumers through a collective yet gradual cut back of sodium levels in ...








/Food-label-Envision-575f13f25f9b58f22ee9a2dc.jpg)
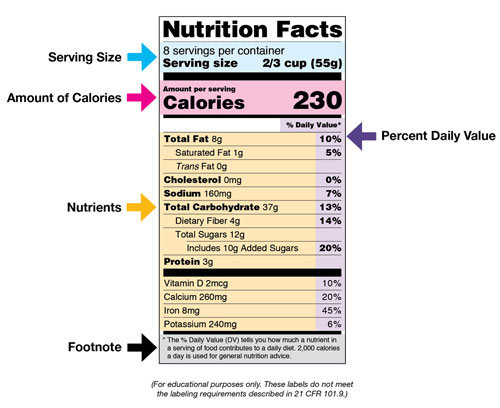
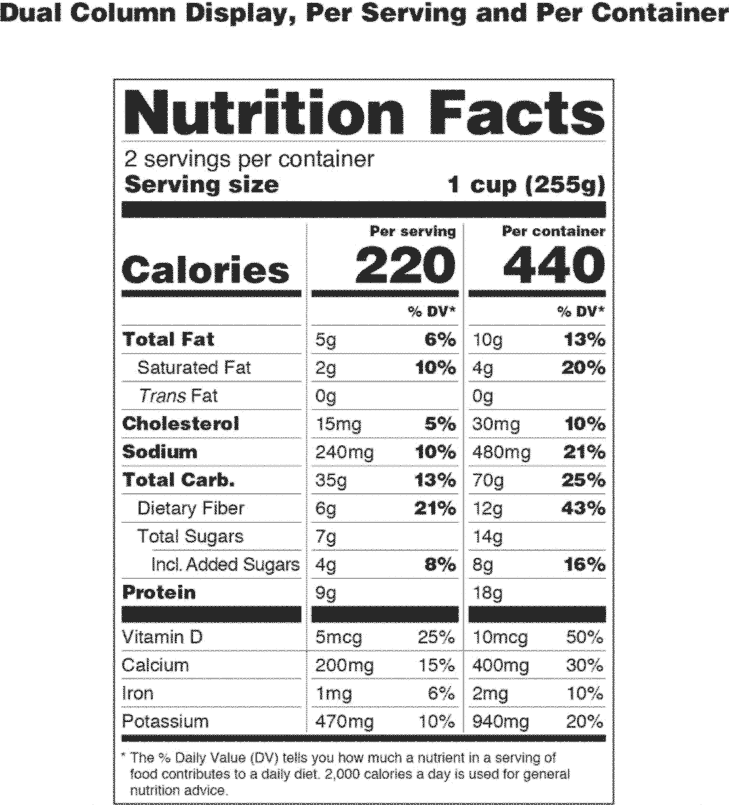



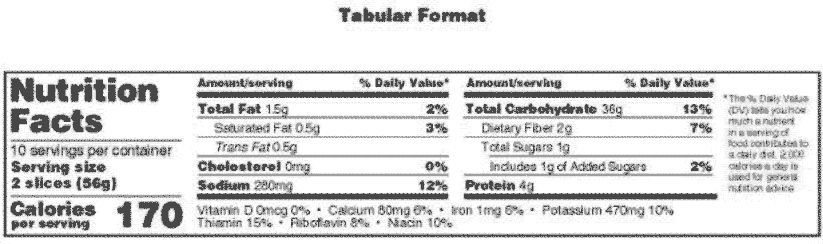
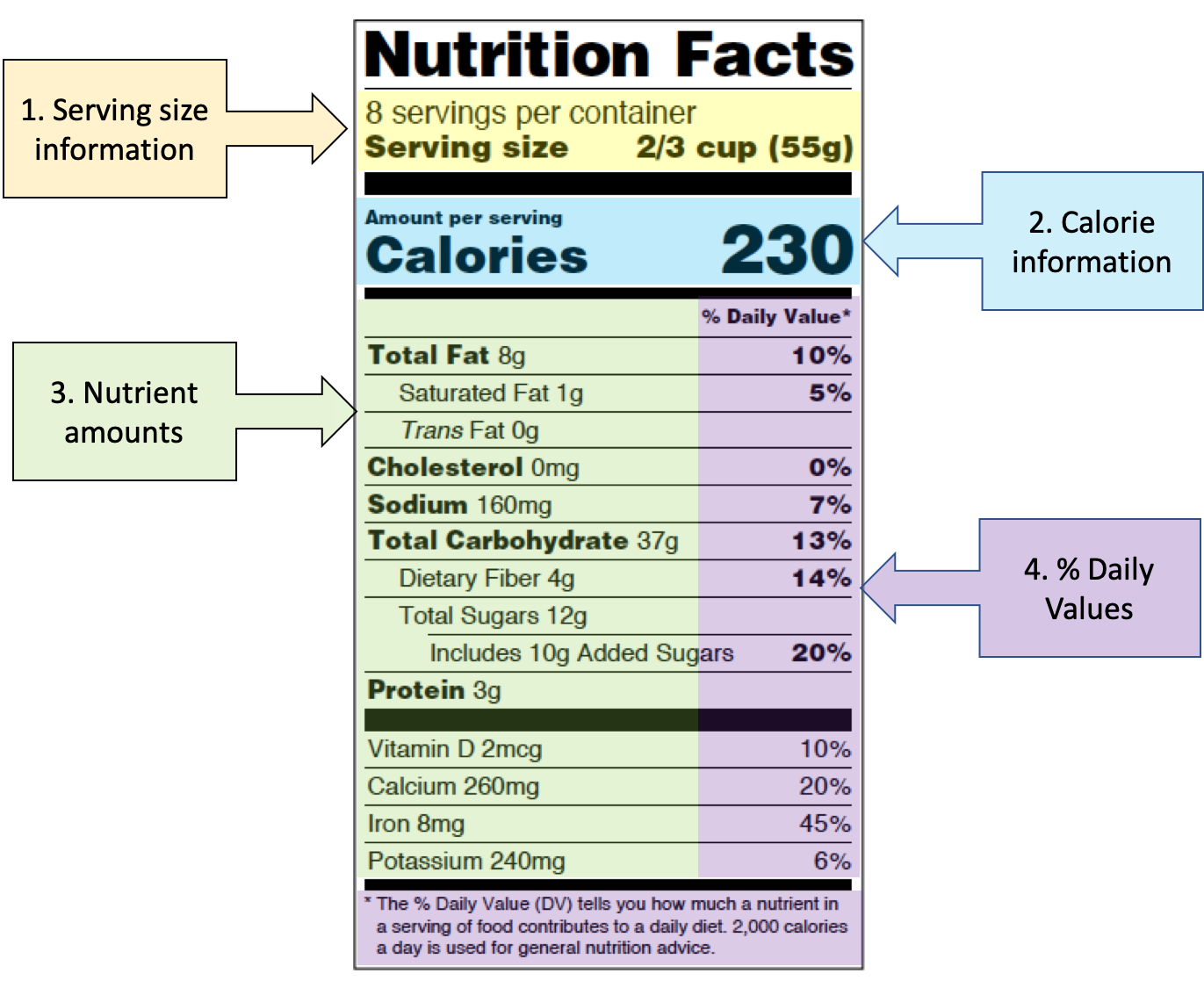













Post a Comment for "44 fda approved statements about food components on food labels"